Premium Only Content
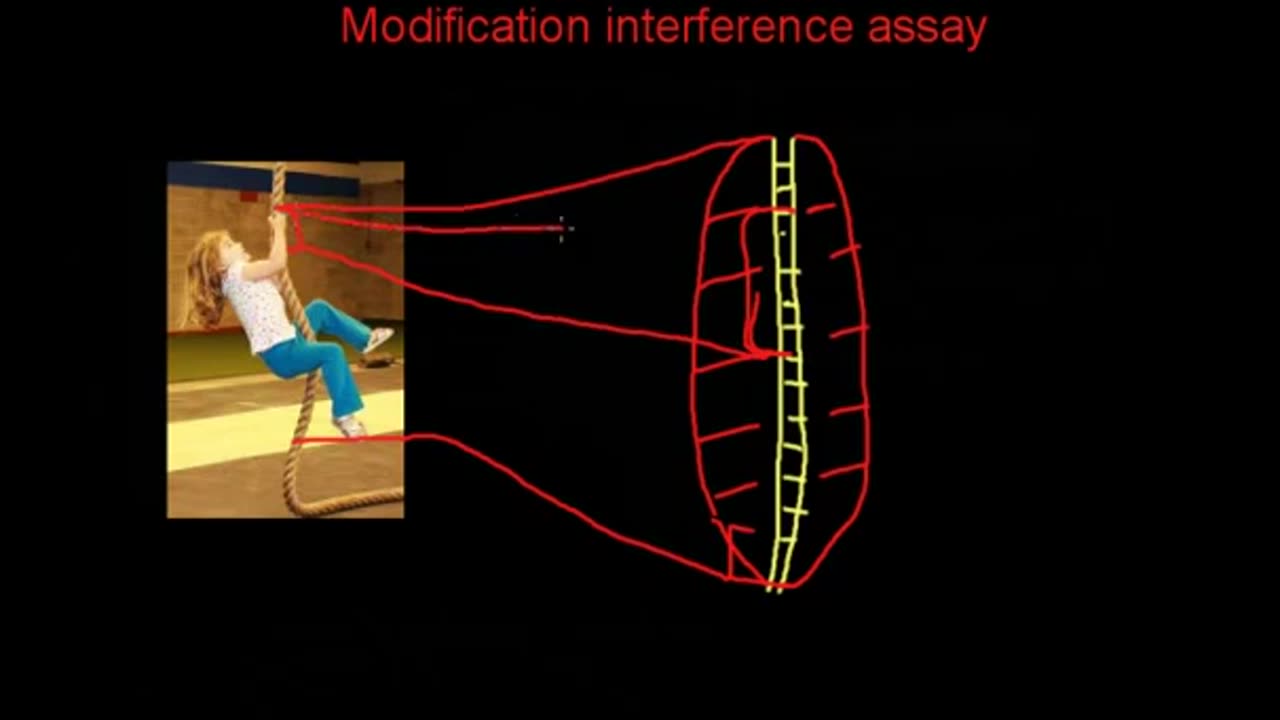
Modification interference assays
Methylation & Uracil Interference Assays
Both the methylation and uracil interference assay analytical methods are presented in parallel because they assay for similar information based on similar reactions. In both cases, the DNA is analyzed for nucleotides which are important for protein binding. The approach taken is to end label the DNA probe so that cleavage of the DNA will yield labeled fragments whose size indicates the cleavage position, as in DNase I footprint analysis. The probe is treated to generate modified bases at about 1 base per DNA molecule. Binding protein is added to the modified DNA. If the base which was modified on a given DNA molecule was critical for binding, that molecule will be left unbound. Bound and unbound populations of DNA are separated on mobility shift assay gels. The two populations are then treated to cleave the DNA at the modified bases and run on a denaturing PAGE gel.
Modifications to bases important for protein binding will lead to the absence of cleavage fragments ending at such bases from the bound fraction of DNA. Cleavage at these sites will produce fragments seen only in the unbound DNA.
Methylation and uracil interference techniques differ in the base(s) targeted, and in the method used to modify and cleave the DNA. The methylation interference assay is the simpler of the two, involving a chemical modification of guanines and adenines with Dimethylsulfate to produce N-7 methyl G or N-3 methyl A residues. These residues are subject to cleavage by piperidine. The complexity of this method is somewhat increased by the need to isolate an end labeled probe with which to work.
In the uracil interference analysis DNA is synthesized in the presence of dUTP to incorporate Uracil residues in place of thymine, at a rate of 0.5-1 thymine substitutions per molecule. This can be accomplished by PCR with one labeled primer, thus probe generation may be easier than for methylation interference. Cleavage at uracil residues requires a two step procedure, in which uracil glycosylase removes the Uracil base, creating apyrimidinic sites which are then cleaved by piperidine.
Methylation Interference Assay
Uracil Interference Assay
Probe Preparation
Generate a probe labeled at one end from a plasmid construct digested with enzymes to produce one 5' overhang on a probe of 100-300 bp. (see protocol on DNase I footprinting)
Prepare and purify fragment by methylating 106 cpm of probe in 200 µl of reaction buffer (50mM sodium cacodylate pH 7.9, 1mM EDTA).
Add 1µl of DMS to start the reaction.
React for 5 minutes at room temperature.
Stop the reaction with 50 µl of the following: 1.5M sodium acetate, pH 7.2, 1mM BME and 0.25 µg/ml tRNA.
Precipitate the DNA with 750µl of ethanol at -70°C (dispose of supernatant as DMS toxic waste).
Wash pellet by redissolving in 300µl 0.3M sodium acetate, then precipitate with 900 µl ethanol. Repeat this step twice.
Proceed to mobility shift analysis.
Probe for this procedure is generated by PCR amplification in the presence of one labeled and one unlabeled primer, with dUTP present at 25% of the concentration of the dTTP. Primers should be selected to provide a region of amplification which is 200-300 bp in length, and contains the protein binding site.
Label the primer(s) with 32P ATP and polynucleotide kinase. Use the buffer and protocol recommended by the enzyme manufacturer.
Purify the probe by gel electrophoresis or spin columns prior to use.
Purify the PCR products by native gel electrophoresis.
Proceed to mobility shift analysis.
Mobility Shift Analysis
Mobility shift analysis is the same for both protocols. At the end of the analysis, locate the bands by autoradiography, and cut out the bound (upper) and unbound (lower) DNA bands. Purify the DNA from the gel slices.
Cleavage Reactions
Uracil glycosylase
Remove the uracil bases by treating the DNA with 0.02 U/µl uracil glycosylase in 1X Taq polymerase buffer (from PCR reaction)
Incubate at 37°C 1 hour
Precipitate DNA with 0.1 vol 0.3M sodium acetate and 3 volumes of ethanol
Proceed with piperidine cleavage.
Piperidine Reaction: (both assays)
Redissolve precipitated DNA in 0.1 ml of 1M piperidine.
Heat to 95°C for 30 minutes. Remove tubes to -70°C.
Lyophilize frozen samples to dryness.
Redissolve in 0.1 ml water.
Repeat lyophilization and rehydration three times.
Analyze bound and unbound samples on a 6% denaturing polyacrylamide gel.
-
 1:05:21
1:05:21
BonginoReport
5 hours agoBratty Protestors F*ck Around & Find Out! - Scrolling w/ Hayley (Ep. 236) - 02/16/26
121K41 -
 2:23:34
2:23:34
Steven Crowder
5 hours agoRubio vs. AOC at Munich is a Preview of 2028 & The Future of Western Civilization
406K179 -

Sean Unpaved
2 hours agoThe 2026 Season Is A PROVE IT Season For Lincoln Riley & USC! | UNPAVED
19.3K -
 LIVE
LIVE
Wendy Bell Radio
7 hours agoHow Much Do You Really Want To Know?
5,530 watching -
 1:46:37
1:46:37
The Dan Bongino Show
6 hours agoThe Epstein Files, Guthrie Update, and Obama on Aliens? (Ep. 2453) - 02/16/2026
549K634 -
 33:56
33:56
The Rubin Report
19 hours agoThe Real Reason Conservatives Are Rethinking Ronald Reagan | Presidents Series | Michael Knowles
57.4K20 -
 LIVE
LIVE
LFA TV
19 hours agoLIVE & BREAKING NEWS! | MONDAY 2/16/26
1,919 watching -
 1:51:10
1:51:10
The Mel K Show
3 hours agoMORNINGS WITH MEL K - 2-16-26 -The Old World Order Exposes Itself
30.4K18 -
 1:27:26
1:27:26
The Shannon Joy Show
4 hours ago🔥The Crisis Pattern: ICE Violence, Venezuela & U.S. Vulnerability With Catherine Austin Fitts🔥
24.4K10 -
 1:01:40
1:01:40
Trumpet Daily
3 hours ago $1.41 earnedIt Should Be Presidents’ Month - Trumpet Daily | Feb. 16, 2026
19K3