Premium Only Content
New experimental mRNA trial suspended
Vaccines and Related Biological Products Advisory Committee December 12, 2024 Meeting Announcement
Entire briefing document
https://www.fda.gov/media/184301/download
Pediatric RSV vaccine trial enrollment on hold in US, VRBPAC says
The halt follows a severe respiratory disease safety signal observed in a July 2024 phase 1 trial of Moderna's mRNA-1345 and mRNA-1365 vaccine candidates.
A phase I trial
The effects of the medication on about 20 to 80 healthy volunteers
To evaluating safety and ideal dosage
70% move on to phase II
This study, safety, tolerability, and immunogenicity
Who were the subjects
Children aged younger than 2 years
Respiratory syncytial virus (RSV) -naïve children aged 2 through 5 years
The halt of this mRNA RSV vaccine
Severe respiratory disease safety signal
Safety signal led to study pause
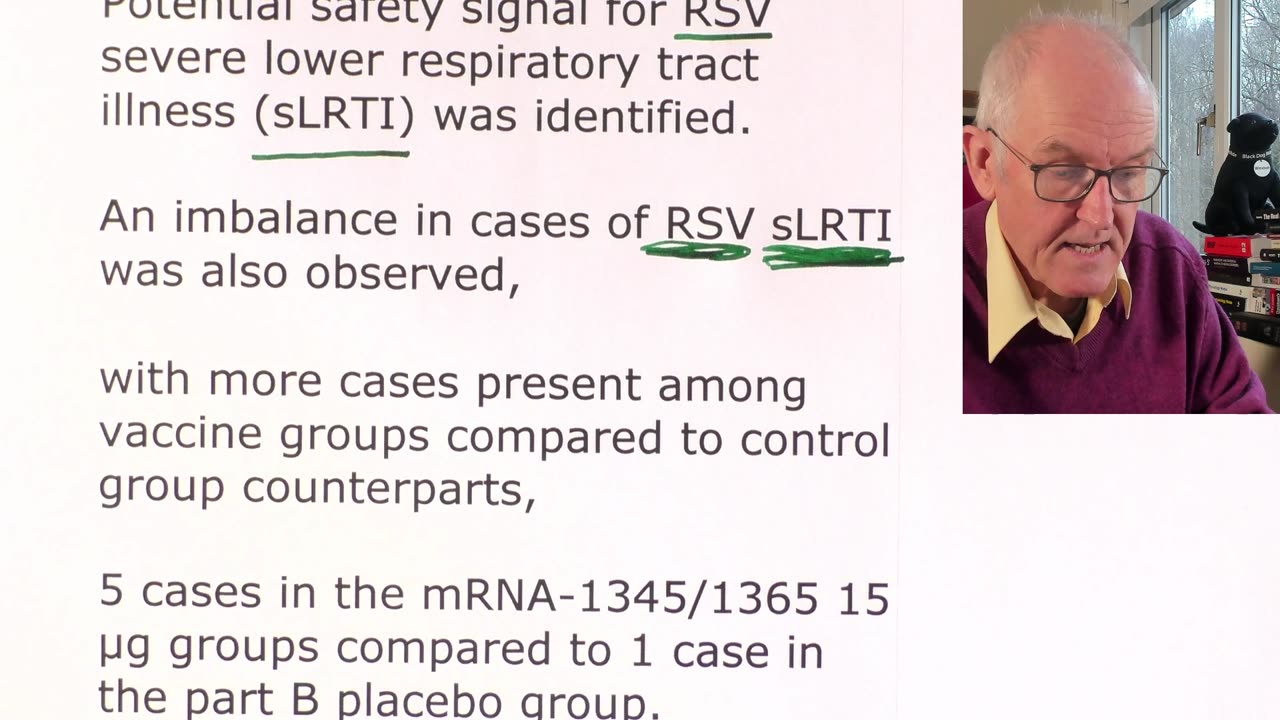
Potential safety signal for RSV severe lower respiratory tract illness (sLRTI) was identified.
An imbalance in cases of RSV sLRTI was also observed,
with more cases present among vaccine groups compared to control group counterparts,
5 cases in the mRNA-1345/1365 15 µg groups compared to 1 case in the part B placebo group.
Of these 6 cases, 5 required hospitalization, including 1 infant who required mechanical ventilation.
which raised concern for vaccine-associated enhanced respiratory disease.
The protocol’s study pause criterion of any sLRTI with positive polymerase chain reaction (PCR) for RSV in ≥2 participants was met.
One, two and three dose parts planned
Part A (Cohorts 1 and 2)
30 µg mRNA-1345, 30 µg mRNA-1365, and placebo
approximately 90 participants 8 months to <24 months of age
(randomized in a 1:1:1 ratio, respectively)."
"Part B (Cohorts 3 through 6)
2 dose levels of mRNA-1345 and mRNA-1365 and placebo
Approximately 120 participants 5 months to <8 months of age
(randomized in a 1:1:1 ratio, respectively)
Part C (Cohorts 7 and 8)
3 doses of 30 µg mRNA-1345
Approximately 100 participants 8 months to <12 months of age
who have (Cohort 7) or have not (Cohort 8) previously received nirsevimab.
VRBPAC meeting and outlook
Vaccines and Related Biological Products Advisory Committee
Vaccine-associated enhanced respiratory disease
Currently considering criteria for recommencement
Scam Accounts
https://www.facebook.com/Campbellteaching/
https://www.facebook.com/dr.john.campbell/
Genuine Campbell Teaching Accounts
https://substack.com/@johninengland?utm_source=user-menu
https://www.paypal.com/mep/dashboard
https://www.paypal.com/donate/?cmd=_s-xclick&hosted_button_id=78GGHGLK5ZXAE
-
 17:26
17:26
Dr. John Campbell
10 days agoIs Nepah visus the next pandemic
12.6K49 -
 LIVE
LIVE
LFA TV
11 hours agoLIVE & BREAKING NEWS! | WEDNESDAY 2/11/26
5,144 watching -
 LIVE
LIVE
Crypto Power Hour
11 hours ago $0.05 earnedNFT The Movie, The Circus Is In Town With Jeff Crane & Miriam Reza
348 watching -
 54:08
54:08
Amy Dangerfield
1 day ago $11.05 earnedIyah May: Israel, Faith & Demonic Music Industry
79K29 -
 5:48
5:48
China Uncensored
16 hours agoThis Was Too Sophisticated To Be An Accident
5.15K8 -
 26:53
26:53
Uncommon Sense In Current Times
17 hours ago $0.22 earnedCompelled Speech vs. Cheap Grace | The Legal & Cultural War on Biblical Truth
5.64K3 -
 LIVE
LIVE
BEK TV
23 hours agoTrent Loos in the Morning - 2/11/2026
181 watching -
 1:58:28
1:58:28
MG Show
21 hours agoRenee Good’s Father-in-Law: Tim Macklin’s Fight for His Grandson & National Revival
64.1K18 -
 1:02:34
1:02:34
Coin Stories with Natalie Brunell
19 hours agoBitcoin Heading to $40K? Kevin Wadsworth Explains the Capital Rotation Event and Gold's Bull Market
17.5K17 -
 16:17
16:17
DeVory Darkins
22 hours agoIlhan Omar makes GRAVE MISTAKE after Congress launches SHOCKING probe
33.9K46