Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
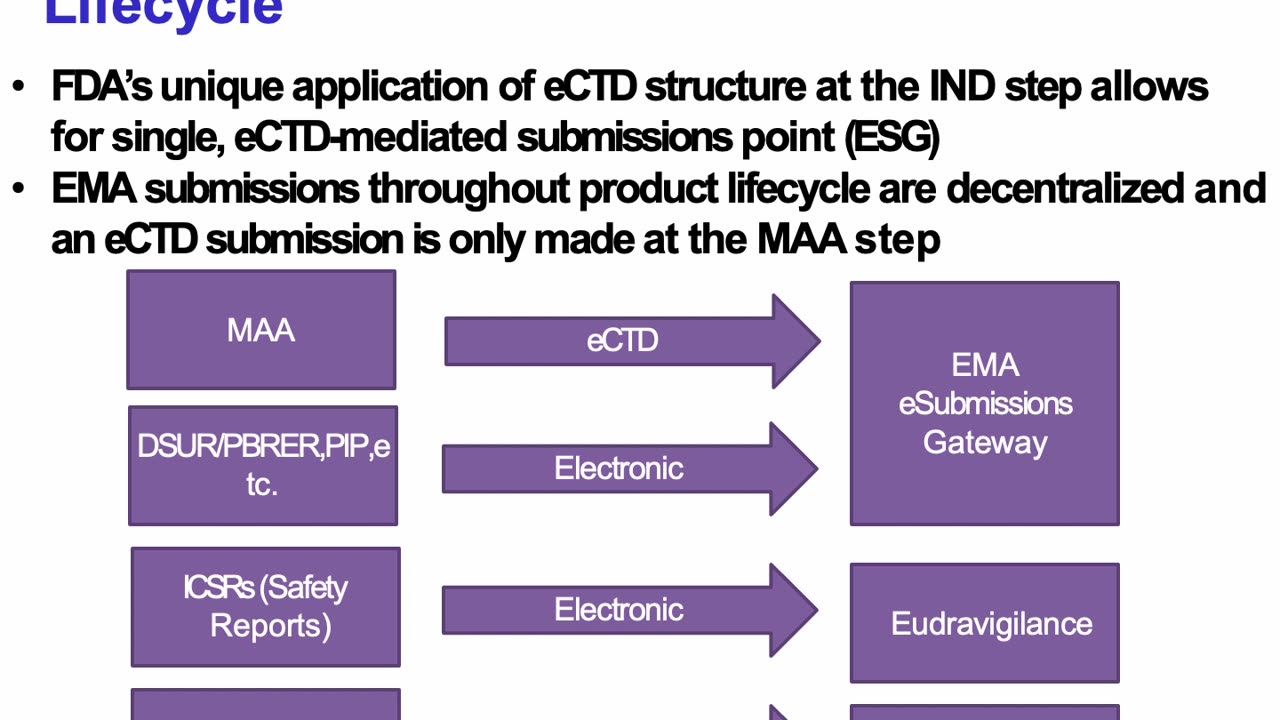
Regulatory Affairs - eCTD Submission Gateways for FDA and EMA, and ICH-M8 (Pharmaceutical Sciences and Health Care products). Peivand Pirouzi, Ph.D.
10 months ago
26
Discover the intricacies of submission gateways for regulatory compliance with the FDA and EMA in this comprehensive guide. In this video, we delve into the ICH-M8 guidelines, exploring how they streamline electronic submission processes for pharmaceutical and biotechnological products. Learn about:
Key features of the FDA’s Electronic Submission Gateway (ESG) and the EMA’s eSubmission Gateway/Web Client.
The role of ICH-M8 in harmonizing electronic standards across regulatory agencies.
Loading 1 comment...
-
 20:19
20:19
RiftTV
21 hours agoKash Patel's GF Is Suing MAGA Influencers for Jokes & Memes | Amy Dangerfield
7.54K19 -
 LIVE
LIVE
StuffCentral
3 hours agoRest here with Stuffy
40 watching -
 LIVE
LIVE
Reidboyy
13 hours ago24/7 BO7 Camo Grind! Stream Doesn't End Until I Unlock EVERY Camo in Black Ops 7!
48 watching -
 23:46
23:46
iCkEdMeL
1 hour ago $1.18 earned🔴 Anna Kepner Case: 3AM Warning and Cabin Screaming Reveal Terrifying Timeline
2.36K -
 LIVE
LIVE
HomieQuest
4 hours agoLive Streaming!
53 watching -
 26:47
26:47
Degenerate Jay
5 hours ago $2.51 earnedPlaying Fallout 3 For The First Time Ever - Here's My Thoughts
12.6K3 -
 LIVE
LIVE
ItsMossy
1 hour ago🍃DEVS ARE CRACKING DOWN🍃maybe a video?🍃shrooms aren't real dude🍃ARC RAIDERS RAIDERING SO HARD🍃
35 watching -
 59:25
59:25
Jeff Ahern
4 hours ago $9.74 earnedThe Saturday Show with Jeff Ahern
75.7K16 -
 29:53
29:53
Afshin Rattansi's Going Underground
2 days agoBRICS MUST Replace the US’ Authoritarian International Financial System! (Prof. Steve Keen)
10.3K16 -
 31:27
31:27
Robbi On The Record
3 days ago $11.40 earnedWhat the Bible say about Astrology.. The Conversation Culture Has Been Avoiding | ft. JT Follows JC
44.9K12