Premium Only Content
Regulatory Affairs - Applications of QSR for Medical Devices: 21 CFR Part 820 and ISO 13485 by Peivand Pirouzi, Ph.D.
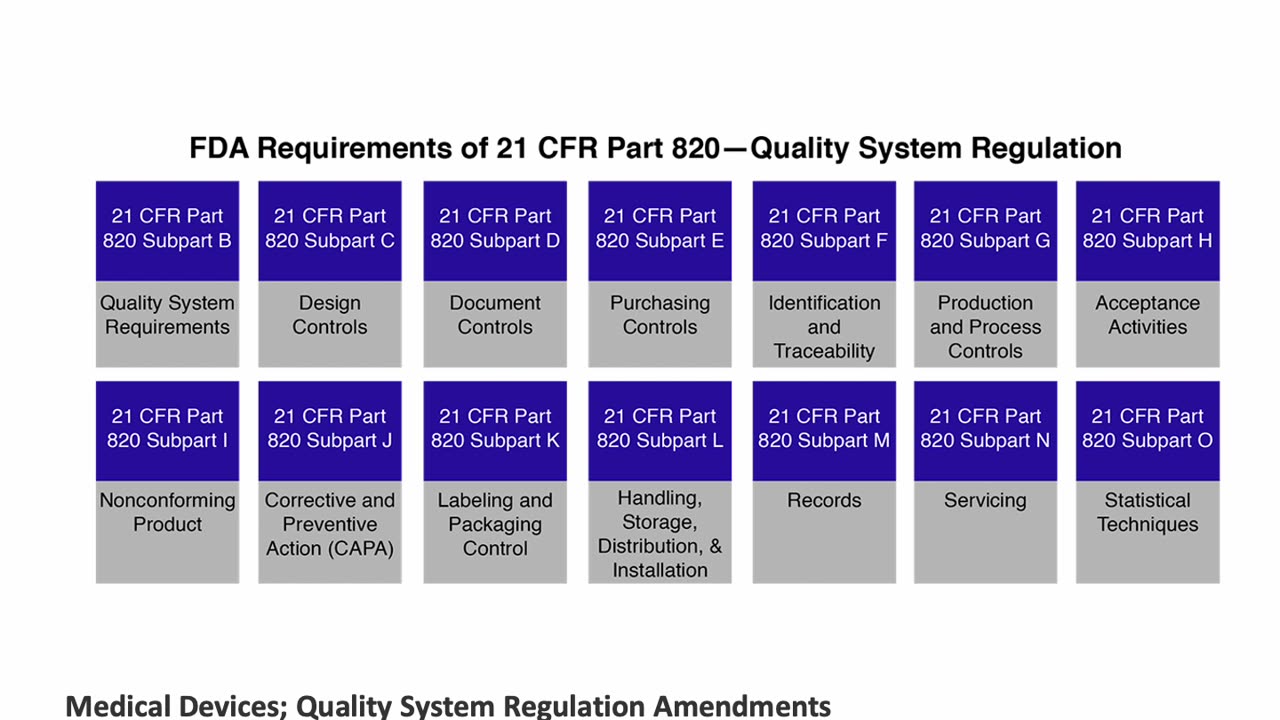
Explore the practical applications of Quality System Regulation (QSR) for medical devices in this insightful presentation by Peivand Pirouzi, Ph.D. This video focuses on the requirements of 21 CFR Part 820, the FDA’s framework for medical device quality systems, and ISO 13485, the global standard for quality management in the medical device industry. Special attention is given to the harmonization of 21 CFR Part 820 with ISO 13485, simplifying compliance for organizations operating in both domestic and international markets.
Professor Pirouzi provides an in-depth analysis of these regulations, offering guidance on implementation strategies to enhance compliance, product safety, and efficiency. Whether you’re navigating domestic requirements or aiming for corporate-level online training and certification by Crown College of Canada (www.crowncollege.ca), this session equips you with the knowledge to succeed.
-
 1:12:09
1:12:09
MattMorseTV
3 hours ago $30.28 earned🔴Trump just GUTTED the ENTIRE SYSTEM. 🔴
38.9K43 -
 LIVE
LIVE
Badlands Media
23 hours agoAltered State S4 Ep. 7
2,426 watching -
 14:37
14:37
World2Briggs
8 hours ago $0.55 earnedTop 10 States Americans Regret Moving To
2.16K4 -
 1:07:59
1:07:59
TheCrucible
5 hours agoThe Extravaganza! EP: 73 (12/10/25)
245K47 -
 1:47:00
1:47:00
Redacted News
6 hours agoBOMBSHELL! NATO'S WORST NIGHTMARE IS ABOUT TO COME TRUE & CONGRESSMAN MASSIE JUST WENT ALL IN
152K193 -
 12:26
12:26
The Gun Collective
3 hours agoDid Glock Copy Itself? NEW GUNS JUST RELEASED!
18.9K5 -
 13:09:11
13:09:11
LFA TV
23 hours agoLIVE & BREAKING NEWS! | WEDNESDAY 12/10/25
220K30 -
 2:13:25
2:13:25
TheSaltyCracker
2 hours agoPodcaster Civil War ReeEStream 12-10-25
43.3K86 -
 1:16:31
1:16:31
Kim Iversen
5 hours agoTucker: Egyptian Planes Trailed Erika Kirk for Years
47.6K95 -

Mally_Mouse
1 day ago🎮 Let's Play!!: Stardew Valley pt. 36
23.5K3