Premium Only Content
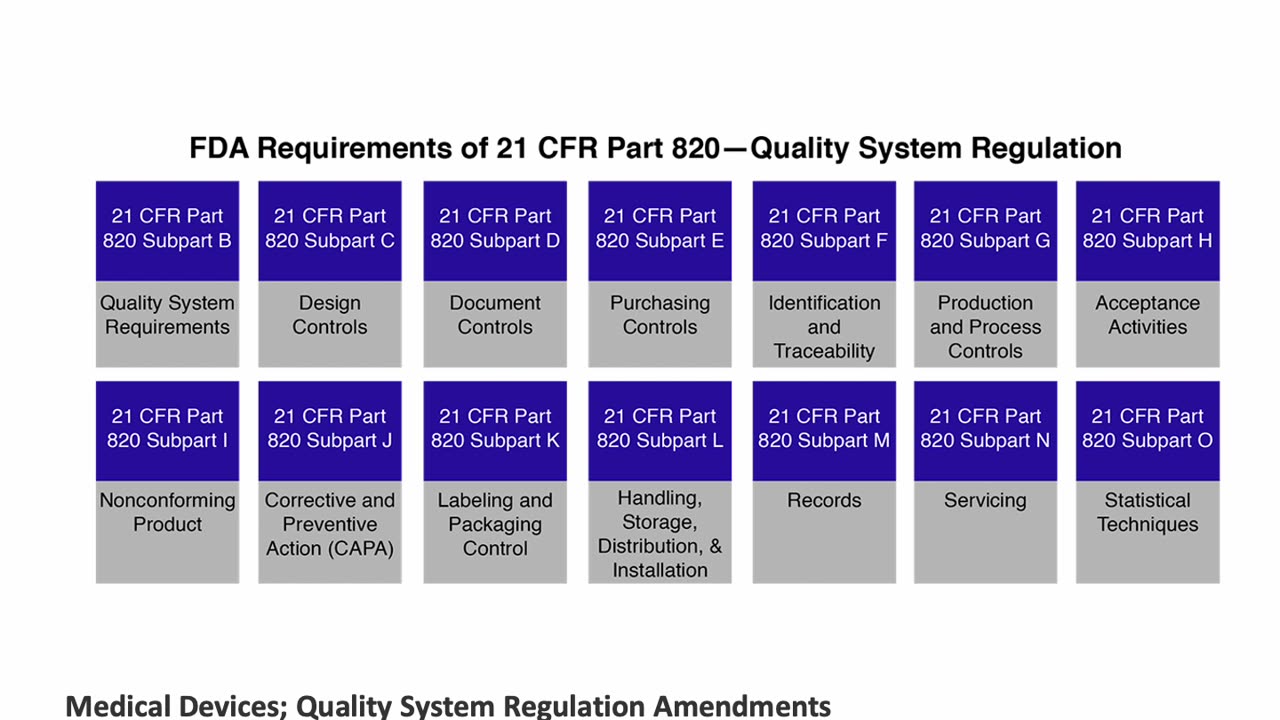
Regulatory Affairs - Applications of QSR for Medical Devices: 21 CFR Part 820 and ISO 13485 by Peivand Pirouzi, Ph.D.
Explore the practical applications of Quality System Regulation (QSR) for medical devices in this insightful presentation by Peivand Pirouzi, Ph.D. This video focuses on the requirements of 21 CFR Part 820, the FDA’s framework for medical device quality systems, and ISO 13485, the global standard for quality management in the medical device industry. Special attention is given to the harmonization of 21 CFR Part 820 with ISO 13485, simplifying compliance for organizations operating in both domestic and international markets.
Professor Pirouzi provides an in-depth analysis of these regulations, offering guidance on implementation strategies to enhance compliance, product safety, and efficiency. Whether you’re navigating domestic requirements or aiming for corporate-level online training and certification by Crown College of Canada (www.crowncollege.ca), this session equips you with the knowledge to succeed.
-
 1:09:10
1:09:10
Game On!
20 hours ago $1.28 earnedFINALLY! MLB Postseason IS HERE!
34.6K2 -
 10:29
10:29
Ken LaCorte: Elephants in Rooms
18 hours ago $2.59 earnedWhy Did Britain Protect Child Molesters?
37.8K19 -
 8:19
8:19
Adam Does Movies
1 day ago $1.06 earnedOne Battle After Another - Movie Review
13.1K2 -
 39:24
39:24
NAG Daily
15 hours agoThe Rezendes Rundown Ep. 21 - National Distress
12K1 -
 2:01:19
2:01:19
BEK TV
1 day agoTrent Loos in the Morning - 9/30/2025
11.2K3 -
 LIVE
LIVE
The Bubba Army
23 hours agoTrump & Netanyahu Done Deal? - Bubba the Love Sponge® Show | 9/30/25
1,622 watching -
 9:15
9:15
ThinkStory
1 day ago6 INSANE Cipher Theories!
22.5K1 -
 20:54
20:54
Jasmin Laine
18 hours ago"Why Are You AVOIDING Me?"—Poilievre GRILLS Carney as He CRUMBLES Under Pressure LIVE
23.8K29 -
 7:13
7:13
China Uncensored
20 hours agoChina’s Military Is Out of Control. Can This INSANE Plan Stop It?
21.7K20 -
 1:46
1:46
WildCreatures
17 days ago $1.37 earnedButterfly risks its life to drink crocodile tears in the Pantanal
20K9