Premium Only Content
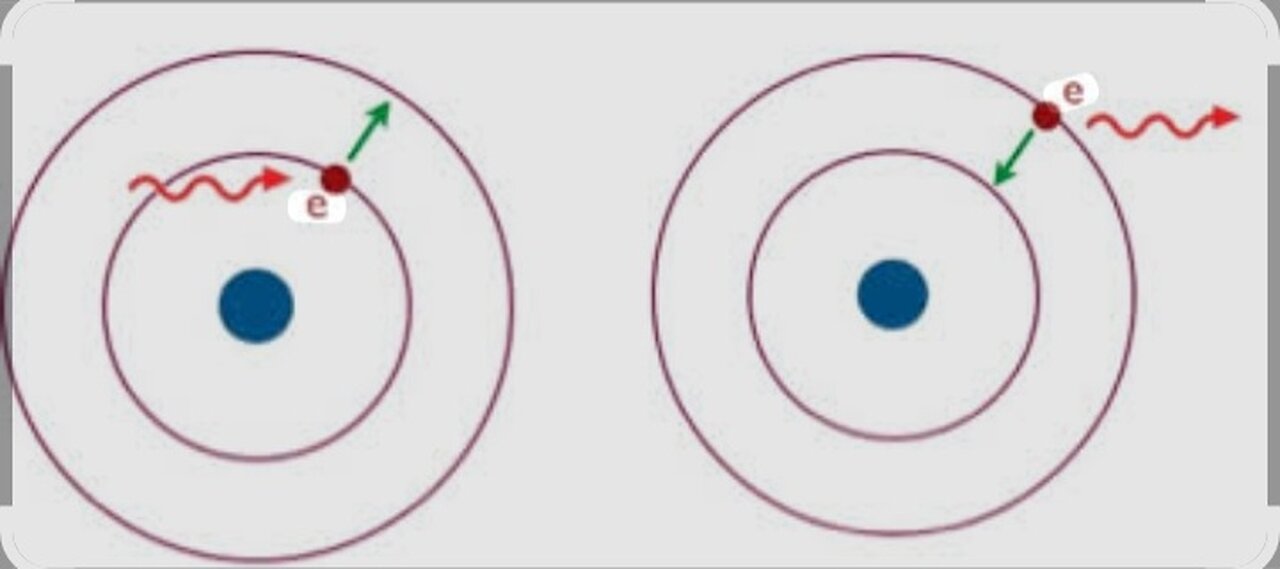
THE ABSORPTION AND EMISSION
The absorption and emission of electrons is a fundamental process in quantum physics that describes how electrons interact with energy.
*Electron absorption*
Electron absorption occurs when an electron in an atom or molecule absorbs energy from an external source, such as light or an electromagnetic field. This energy can come from:
1. *Photons *: Photons absorption can excite electrons to higher energy levels.
2. *Electromagnetic fields *: Electromagnetic fields can transfer energy to electrons.
When an electron absorbs energy, it can:
1. *Excue *: Jump to a higher energy level.
2. *Ionize *: escape from the atom or molecule.
*Electron emission*
The emission of electrons occurs when an electron in an atom or molecule releases energy and returns to a lower energy level. This energy can be released in the form of:
1. *Photons *: The photon emission can occur when an electron returns to a lower energy level.
2. *Heat *: Energy can also be transferred to other degrees of freedom, such as vibration or molecular rotation.
The emission of electrons can occur in several ways:
1. *Spontaneous emission *: The broadcast of photons without external stimulus.
2. *Stimulated emission *: The issuance of photons due to interaction with an external electromagnetic field.
*Applications*
1. *Lasers *: The stimulated emission is essential for the operation of the lasers.
2. *Particle detectors *: The absorption and emission of electrons is used in particle detectors to measure energy and quantity of particles.
3. *Spectroscopy *: The absorption and emission of electrons is used in spectroscopy to analyze the structure and properties of atoms and molecules.
-
 1:04:54
1:04:54
A Cigar Hustlers Podcast Every Day
1 day agoHustlers Podcast Every Week Day Episode 429 "Two Icons"
12.7K -
 LIVE
LIVE
BEK TV
23 hours agoTrent Loos in the Morning - 12/16/2025
151 watching -
 47:05
47:05
Athlete & Artist Show
10 days ago $0.67 earnedHIGH STAKES w/ Former Team Canada Gold Medalist
14.7K -
 2:53
2:53
GreenMan Studio
14 hours agoGREENMANS STOCKING STUFFERS 2 – GRIMMS CAMPING SUPPLIES
14.3K4 -
![Special guest: Sam Anthony, CEO & Founder, [your] News](https://1a-1791.com/video/fwe2/16/s8/1/q/B/l/I/qBlIz.0kob-small-Special-guest-Sam-Anthony-C.jpg) 42:06
42:06
Rpurham
21 hours agoSpecial guest: Sam Anthony, CEO & Founder, [your] News
14.4K -
 15:23
15:23
Standpoint with Gabe Groisman
19 hours agoDual Citizenship Coming to an End? US Senator Bernie Moreno
100K25 -
 1:22:19
1:22:19
FreshandFit
12 hours agoGirls Try To Get 60 Year Old Granny To Do OF
372K139 -
 3:05:53
3:05:53
Decoy
12 hours agoNobody is talking about this..
102K28 -
 1:57:00
1:57:00
Badlands Media
18 hours agoBaseless Conspiracies Ep. 163: False Memories, MKUltra & the Machinery of Disbelief
95.2K24 -
 5:34:44
5:34:44
Drew Hernandez
1 day agoERIKA KIRK & CANDACE OWENS CEASEFIRE SUMMIT?
61K34