Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
Pfizer: Serious and Non-Serious Adverse Reactions from Post-Marketing Data
2 days ago
66
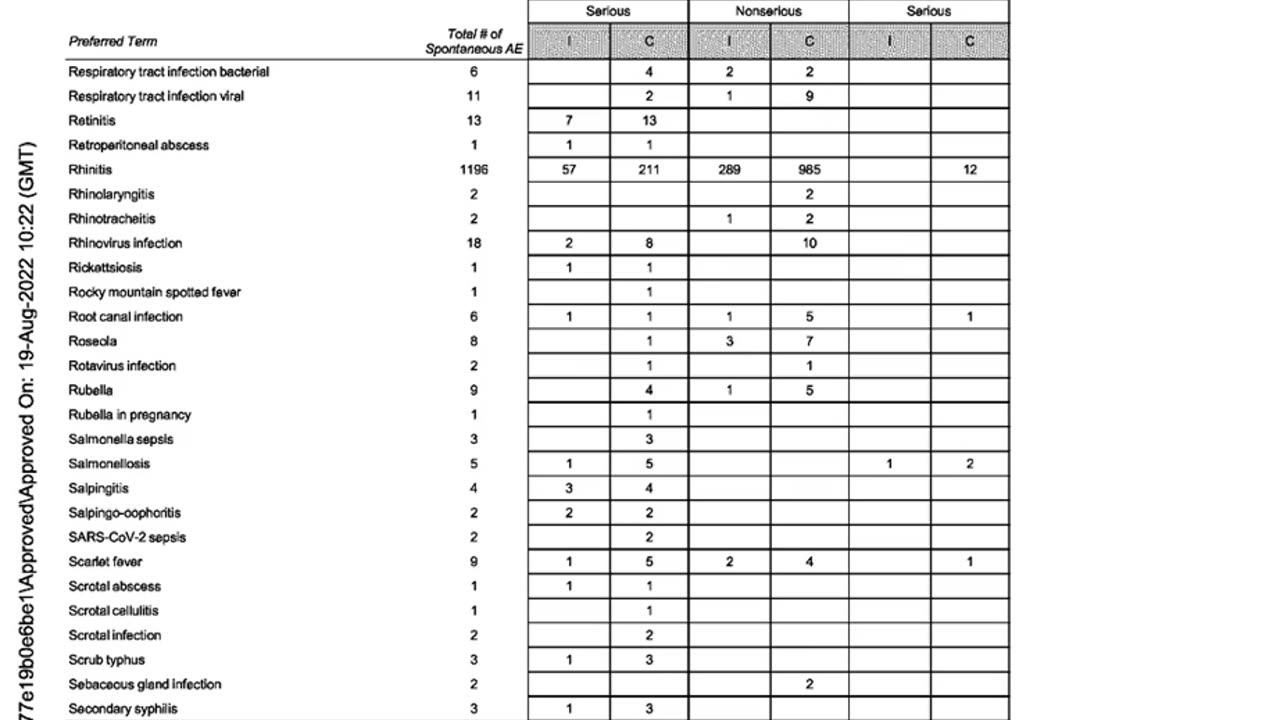
APPENDIX 2.2: Cumulative and Interval Summary Tabulation of Serious and Non-Serious Adverse Reactions from Post-Marketing Data Sources
BNT162B2
Cumulative Reporting Period: Through 18-JUN-2022
Interval Reporting Period: 19-DEC-2021 Through 18-JUN-2022
Total Number of Cases: 507,683 (Interval) / 1,485,027 (Cumulative)
Total Number of Adverse Events (PT): 1,591,026 (Interval) / 4,964,106 (Cumulative)
MedDRA Version: v.25.0J
Loading 1 comment...
-
 0:40
0:40
Canadian Citizens Journal
5 hours ago🤬This is not ok! Not for young kids to know! Copied from Gays Against Groomers
19 -
 LIVE
LIVE
Russell Brand
2 hours agoGavin de Becker | Fear, Freedom & Resisting Control - SF642
1,792 watching -
 10:34
10:34
Clownfish TV
3 hours agoGetting 'Canceled by Trump' is GREAT for Ratings?! | Clownfish TV
8074 -
 14:42
14:42
Professor Gerdes Explains 🇺🇦
2 hours agoThis ONE SENTENCE from Putin Guarantees a Long War
17 -
 19:05
19:05
Sponsored By Jesus Podcast
2 days agoI Lost the World But Gained My SOUL | Freedom in Christian Suffering
1.14K3 -
 14:47
14:47
Dr. Nick Zyrowski
15 days agoFasting Is THE Cure - NO FOOD FOR 3 DAYS Completely Heals You!
1.35K9 -
 LIVE
LIVE
ROSE UNPLUGGED
51 minutes agoClimate Fatigue: Is the Whole World Feeling It?
66 watching -
 2:01:24
2:01:24
The Charlie Kirk Show
2 hours agoBiblical Borders + The Illegal Superintendent + Shutdown Fever | Deace, Homan | 9.30.2025
184K39 -
 LIVE
LIVE
Badlands Media
11 hours agoGeopolitics with Ghost Ep. 42
1,157 watching -
 2:01:33
2:01:33
Right Side Broadcasting Network
3 hours agoLIVE REPLAY: President Trump Makes an Announcement - 9/30/25
108K29