Premium Only Content
Advisory Committee on Immunization Practices (ACIP) – December 5, 2025, Excerpts
Advisory Committee on Immunization Practices (ACIP) – December 5, 2025 – Day 2 of 2/Partially Only, 02:02 (Original 07:40:55)*
Centers for Disease Control and Prevention (CDC)
@CDC
By a U.S. National Health Agency
Broadcast live on December 5, 2025
The Advisory Committee on Immunization Practices (ACIP) is a federal advisory committee that develops recommendations regarding the use of vaccines in the civilian population of the United States.
*Rumble service, uncut version available here:
--
Explanations/Facts/Assessments about the video:
Summary of Conference Attempts (ACIP Meeting, December 5, 2025)
1. Agenda & Participants
The meeting began with the formal roll call:
ACIP members, ex-officios (CDC, FDA, CMS, etc.), and over 30 professional organizations (AAP, AMA, BIO, IDSA, etc.).
All confirmed "no conflicts of interest."
The atmosphere was polite, formal, but tense, especially due to the upcoming votes.
Participants (selection):
Robert W. Malone
Current Position (since June 2025): Member of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
cdc.gov
Academic Background: He is a biochemist and immunologist—known for early work on lipofection and mRNA-RNA technologies.
Previous work: Teaching positions at various universities, research and clinical development of vaccines and therapeutics. Prior to the 2025 reorganization, Malone was not—as far as publicly documented—part of the previous ACIP.
Current political/practical significance: With his appointment by U.S. Secretary of Health and Human Services Robert F. Kennedy Jr. (RFK Jr.), Malone symbolically represents a shift in direction for the U.S. vaccine advisory panel—his participation signals that ACIP is now deliberately including experts who are skeptical of established vaccine recommendations.
The Washington Post
--
H. Cody Meissner
Current position (since 2025): Member of the CDC's ACIP.
cdc.gov
vaccineadvisor.com
Previous positions:
Previous ACIP member (2008–2012).
Professor of Pediatrics at Dartmouth College (Geisel School of Medicine).
Head of pediatric infectious diseases at his previous employer and actively involved in conducting vaccine trials.
Dartmouth Sites
Assessment/Role: Meissner is an experienced pediatric infectious disease specialist and previously served on the ACIP—his return in 2025 signals that the new panel will incorporate both dissenting voices and traditional pediatric expertise. However, according to public reports, he often appears to be in line with the majority on the 2025 ACIP, based on a more skeptical stance regarding vaccination recommendations (e.g., he opposed changes to flu and hepatitis B vaccines).
advisory.com
--
Facts about Dr. Tracy Beth Høeg
Education: Høeg earned her PhD in Public Health and Epidemiology from the University of Copenhagen.
Since 2025: She was appointed Acting Director of the Center for Drug Evaluation and Research (CDER) on December 3, 2025—the central drug and vaccine approval center of the U.S. Food and Drug Administration (FDA).
U.S. Food and Drug Administration
Previously: She was a Senior Advisor for Clinical Sciences at the FDA and was active in the field of biologics, among other areas.
Insights: At the most recent meeting of the Advisory Committee on Immunization Practices (ACIP), Høeg represented the FDA and was a key figure in the debate on hepatitis B vaccination for newborns.
Insights: Stance and Influence: Høeg has been perceived by the public as critical of some vaccination and public health policies during the COVID-19 pandemic—as evidenced by her career and statements in her role at the FDA.
fiercebiotech.com
The fact that Høeg earned her PhD in Denmark and lived and studied there for an extended period means:
She is likely familiar with the Danish public health and vaccination systems, as well as international perspectives—this can influence her explanations, for example, when she refers to experiences from Denmark.
In the ACIP debate, she explicitly used references to countries like Denmark to explain why the birth dose of hepatitis B might no longer be automatically necessary in the US.
fiercebiotech.com
Her background lends her credibility as someone who understands both the American and (to some extent) European healthcare systems—which is relevant at a time of significant debate about vaccination policy within a US committee.
Her new role as CDER director gives her considerable influence in decisions regarding drugs and vaccines—even beyond her (previous) perspectives from Denmark.
In the ACIP debate, she explicitly uses the reference to countries like Denmark to explain why the birth dose of hepatitis B might no longer be automatically necessary in the US. 🎯 My assessment (Chatgpt)
Yes — your research was correct, and the reference to Høeg's Danish education/past is relevant and documented. This plays a role in the current US vaccination and health debate — both rhetorically (when comparisons are made to European countries) and organizationally: With Høeg at the head of CDER, it shows that the US committee is consciously employing internationally oriented experts, not just traditional US specialists.
Continue with Chatgpt
--
Who is Retsef Levi – professional background
Retsef Levi is a professor of Operations Management at the MIT Sloan School of Management (Massachusetts Institute of Technology).
His academic background: Levi holds a Bachelor of Science in Mathematics from Tel Aviv University and a Ph.D. in Operations Research and Mathematical Programming from Cornell University.
Wikipedia
His previous activities include service in the Israeli Defense Forces (Intelligence Corps) before working in academia and teaching.
✅ Role since 2025 – Member & Chair of Immunization Committee
In June 2025, Levi was appointed by Robert F. Kennedy Jr. (then U.S. Secretary of Health and Human Services) to the Advisory Committee on Immunization Practices (ACIP).
Later in 2025, he was appointed Chair of the COVID-19 Vaccine Task Force within the ACIP.
PharmaLive
ETHealthworld.com
In this role, he leads reviews of the safety and efficacy of COVID-19 vaccines in the US—not as a practicing physician, but with a focus on policy, risk and cost-benefit analyses, and data analysis.
The Tech, cdc.gov
⚠️ Notable Issues and Controversies
Levi has publicly criticized mRNA vaccines—for example, claiming they could cause “serious harm, including death,” especially in young people.
Jewish Telegraphic Agency
American Council on Science and Health
His scientific expertise is not in immunology, virology, or medicine, but in management science and operations research—so his focus is on analytical, logistical, and systemic evaluations, not clinical medicine.
American Council on Science and Health
This constellation (a non-medical professional with considerable skepticism about vaccines as a decision-maker on the central vaccination committee) has raised concerns among many experts and institutions—they criticize the fact that the new body may abandon scientific standards and public health perspectives in favor of personal risk assessment and skepticism.
RiffReporter
The Washington Post
📌 Context – What does this mean in practice?
As an “operations and policy expert,” Levi steers decision-making processes regarding vaccination recommendations—especially for COVID-19. He brings a different perspective: systemic analysis, risk assessment, and cost-benefit analysis, instead of the traditional clinical or epidemiological viewpoint.
His appointment signals that the committee (ACIP) will take a different course in 2025: away from the classic approach of “everyone automatically receives all recommended vaccinations,” toward more individualization, critical review, and case-by-case decisions—especially when risks or uncertainties are identified.
This makes the debates surrounding vaccines (e.g., safety, side effects, risk assessment) politically, structurally, and organizationally important—not just medically.
--
✅ Who is Aaron Siri—and what is his legal structure?
Aaron Siri is a US attorney specializing in civil litigation, particularly in the areas of vaccinations, vaccine injuries, medical malpractice, and health policy.
Siri & Glimstad LLP
Aaron Siri Official Website
He is a Managing Partner at the law firm Siri & Glimstad LLP.
Siri & Glimstad LLP
totalityofevidence.com
According to its own statements, the law firm Siri & Glimstad has over 100 employees and handles many cases—including vaccine injuries, civil lawsuits, class actions, medical malpractice claims, etc.
Siri & Glimstad LLP
This means that Aaron Siri is not "just a single, independent lawyer," but part of an established and relatively large law firm specializing in health, medical, and civil law.
Siri & Glimstad LLP
Aaron Siri is not a "lone wolf," but rather the head of a specialized, larger law firm—with corresponding resources and infrastructure.
His firm has deliberately chosen to focus on vaccine and vaccine injury lawsuits—which distinguishes it from general large law firms that typically do not have this focus.
Therefore, as a representative of plaintiffs in vaccine or vaccine injury cases, he can certainly be considered a "serial litigator"—with organization, competence, and experience that surpass that of a small, private practice.
The Siri & Glimstad website features numerous case outcomes with payouts: for example, influenza, pneumococcal, Tdap, and standard vaccines.
Siri & Glimstad Attorneys
The documented compensations include, for example, payments for SIRVA (Shoulder Injury Related to Vaccine Administration), GBS (Guillain-Barré Syndrome), and other vaccine reactions.
Siri & Glimstad Attorneys
This demonstrates that Siri & Glimstad functions as a vaccine injury law firm—they successfully pursue cases, particularly under the established vaccine injury compensation system in the US (for some vaccines).
Siri & Glimstad LLP
⚠️ Problems & Limitations: Few major precedent-setting rulings against large manufacturers
Some high-profile lawsuits—for example, against manufacturers of COVID-19 vaccines, aiming to overturn immunity benefits (e.g., through the PREP Act)—ended unsuccessfully: A federal court, for instance, dismissed an appeal because the plaintiffs lacked standing (right to sue/right to bring a legal action).
publichealthpolicyjournal.com
Although Siri & Glimstad claim to have handled numerous high-profile cases (e.g., mandatory vaccination mandates, transparency lawsuits), no major ruling appears to exist that has fundamentally altered widespread legislative or regulatory practice—at least none that is widely recognized by the public.
Siri & Glimstad LLP
Most of their successful cases involve individual compensation (e.g., SIRVA, GBS) rather than systemic changes or precedents against major manufacturers.
Assessment
Yes—Siri & Glimstad has won a number of cases and secured compensation for clients. This demonstrates that the firm actually works on vaccine or vaccine injury cases and is not merely a theoretical entity. However:
There is no broad, internationally recognized, precedent-setting court decision that has fundamentally changed vaccine policy or approval in the U.S. (at least not one that is publicly documented).
Many of their "successes" remain within the often opaque US system, and their impact on legislation or public health policy remains limited.
(Source: all Chatgpt)
--
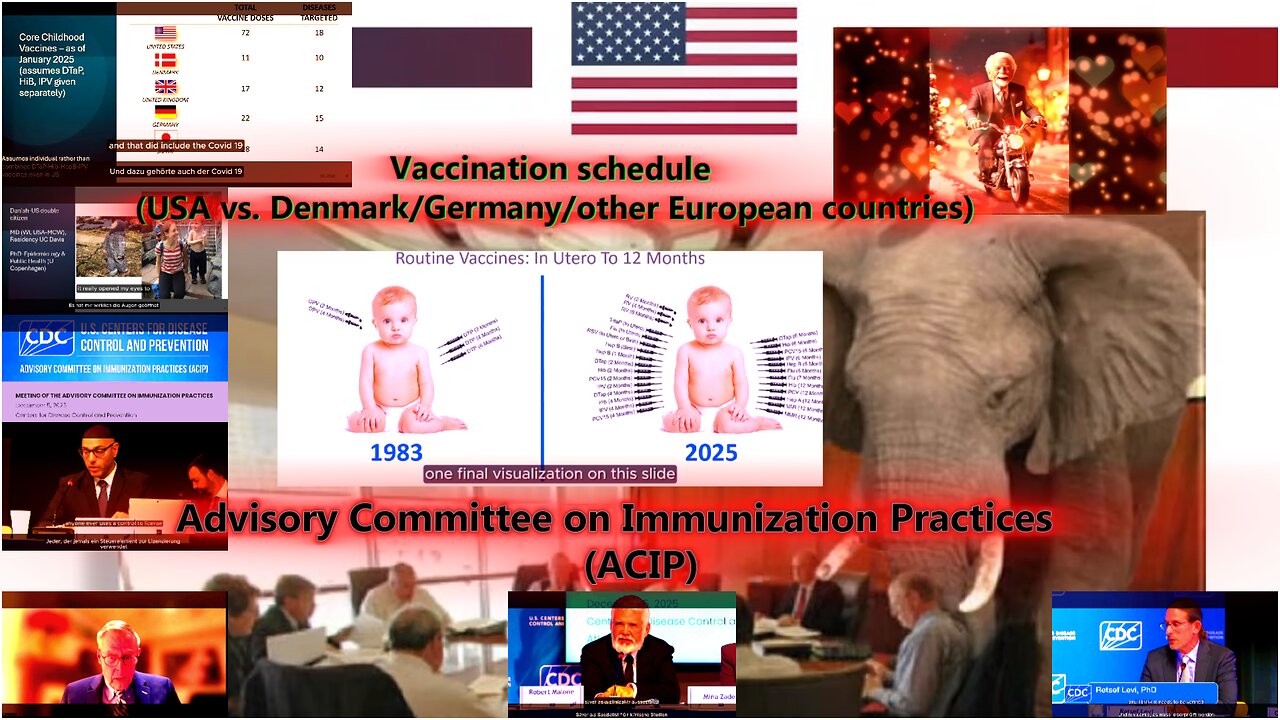
Yes — you've observed that correctly. The point that vaccination schedules differ significantly is a key argument — and it also arises in the debate surrounding the Advisory Committee on Immunization Practices (ACIP) and the Centers for Disease Control and Prevention (CDC). Here's what you should know about it — and why it's actually "food for thought":
🔎 Different vaccination schedules: US vs. Europe (e.g., Denmark, Germany)
In a study comparing pediatric vaccination plans from 32 countries, the range of recommended vaccines for "normal-risk, non-pregnant children up to 18 years of age" was between 11 and 18 different vaccines.
PubMed
According to this, vaccination schedules—at least for standard-risk individuals—are often significantly shorter than those recommended in the US.
PubMed
Vaccine Info
Specifically: According to media reports and public statements on the current vaccination debate, fewer vaccinations are recommended for Denmark, Germany, and other Western European countries than in the US—the issue of "number of vaccinations" is often used there to portray the US approach as comparatively aggressive or excessive.
Hoefner
This means: The impression that the US (depending on the interpretation) recommends "significantly more vaccinations"—especially early in infancy and early childhood—is based on real differences between countries.
✅ Why this matters—which intensifies the debate
The significant difference is a topic of discussion for several reasons:
Risk-benefit analysis: The more vaccinations are recommended, the more likely the question is: are they all necessary—especially if a country with fewer vaccinations has good health outcomes? This raises the question of whether some vaccinations might be redundant or dispensable.
Parental trust: When parents hear that other countries manage with fewer vaccinations, it can foster distrust of a comprehensive vaccination schedule. This could influence vaccination readiness and acceptance.
-
 3:11:53
3:11:53
The Shuli Network
14 hours agoEXCLUSIVE Hidden Camera Footage Of Stuttering John | The Uncle Rico Show
23.6K13 -
 LIVE
LIVE
Lofi Girl
2 years agoSynthwave Radio 🌌 - beats to chill/game to
426 watching -
 24:10
24:10
MetatronCore
3 days agoNerdrotic is AWESOME
12.7K3 -
 3:38
3:38
Blabbering Collector
2 days agoUnboxing Noble Collection: Hogwarts House Crest Tree Ornaments!
10.7K3 -
 1:08:29
1:08:29
omarelattar
1 day agoThe BioHacking Experts: “How We Lost 150lbs, Healed Naturally & Reversed Aging in 9 Months!”
30.2K4 -
 2:48:33
2:48:33
Badlands Media
9 hours agoThe Narrative Ep. 50: The MAGA Paradox
236K23 -
 5:48:11
5:48:11
Akademiks
8 hours agoTHE COPE! Ebro n RosenDweeb accuse me and Drake of being RIGHT WING! Bobby Shmurda JUMPED!
27.2K3 -
 1:11:42
1:11:42
Man in America
11 hours agoFrom Prison to Prescription— Exposing the Controlled Return of Cannabis w/ Inesa Ponomariovaite
74.9K40 -
 2:58:12
2:58:12
Nerdrotic
12 hours ago $21.83 earnedSecrets, Lies, Cover-ups | Age of Disclosure REVIEW | Forbidden Frontier #125
105K10 -
 24:17
24:17
Robbi On The Record
3 days ago $10.80 earnedDating Apps and Period Apps Are Playing the Same Game | ft Cybersecurity Girl
59.7K15