Premium Only Content
Dr. David Wiseman's presentation at the C19 minisymposium session, November 7 2021
Re-analysis of policy-shaping studies on hydroxychloroquine and ivermectin reverses original findings to yield significant benefits. Review in the context of regulatory decisions on vaccines.
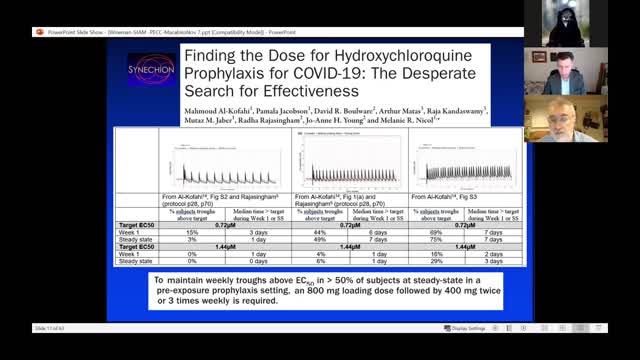
Abstract: It was with skepticism about the efficacy of hydroxychloroquine (HCQ) in Covid-19, that we read a paper reported in NEJM that was one of only two substantive papers cited by FDA in its revocation of the EUA for HCQ in June 2020. This was a study of postexposure prophylaxis (PEP) in persons experiencing a high or moderate risk exposure to Covid-19. Subjects enrolled in the study online and received HCQ or a folate placebo with the goal of preventing development of disease. Data presented in the supplemental appendix suggested, surprisingly, that the drug may have some efficacy in certain subpopulations. After contacting the PI, he pointed out a possible benefit of very early intervention. Exploring this observation, an examination of the raw dataset suggested that the intervention lag had likely been underestimated. After further data requests, we established that $52\%$ of subjects had received drug later than the overnight delivery assumed by the investigators. Correcting for this error yielded a $42\%$ reduction $(p=0.044)$ in Covid-19 compatible disease associated with HCQ given $\leq 3$ days from exposure. We found a similar problem in a companion study performed by the same team under the same logistical arrangements addressing early symptomatic subjects. We were unable to obtain corrected intervention lag data to determine if effects could be observed that were greater than the $20\%$ reduction of symptomatic Covid-19 reported originally. We also re-analyzed a study of early treatment using ivermectin (IVM) reported in JAMA in March 2021. The paper reported several execution errors or changes, notably the dosing of some placebo subjects with active drug, and the use of different types of placebos in different trial phases. Obtaining the raw dataset, accounting for these issues yielded a $56\%$ reduction $(p=0.033)$ of residual Covid-19. We will discuss these studies in the context of significant decisions made during the pandemic, particularly in light of recent decisions made by FDA and CDC regarding the Covid-19 vaccines.
Presented at the "Efficacy and safety statistics of COVID-19 treatment and prophylaxis protocols" minisymposium session at the 4th Annual Meeting of the SIAM Texas-Louisiana Section on November 7, 2021. This presentation does not necessarily represent the opinions or policies of SIAM, but it is protected under Academic Freedom in Research and the 1st Amendment of the United States Constitution.
-
 2:50:01
2:50:01
Eleftherios Gkioulekas -- Research and Commentary
2 years agoExpert testimony to Pennsylvania State Senate, June 2023
224 -
 9:39
9:39
Nicholas Bowling
10 hours ago $0.52 earnedShould Christians Be Pro-Choice Because of Free Will?
9.06K5 -
 2:41
2:41
Canadian Crooner
3 years agoPat Coolen | Jingle Bells
38.4K4 -
 40:40
40:40
Sarah Westall
10 hours agoWhy Curiosity Feels Dangerous in Today’s World — w/ Dr, Debra Clary
18.9K2 -
 58:47
58:47
Flyover Conservatives
23 hours agoWelfare. Ballots. Speech. How the West Criminalized Truth and Protected Corruption | FOC Show
19.7K -
 14:37
14:37
Stephen Gardner
5 hours ago'The Entire PSYOP is COLLAPSING' says Alex Jones! New WARNING Shared from Tulsi Gabbard!!
44.5K63 -
 50:26
50:26
BonginoReport
14 hours agoCalifornia’s Future w/ Sheriff Chad Bianco - Nightly Scroll w/ Hayley Caronia (Ep.203)
117K43 -
 LIVE
LIVE
SpartakusLIVE
6 hours agoDuos w/ Sophie || #1 MACHINE is taking NO DAYS OFF
217 watching -
 3:48:13
3:48:13
RealMetatron
6 hours agoChristopher Nolan's Odyssey is a DISASTER!
34K5 -
 2:40:00
2:40:00
Nikko Ortiz
6 hours agoGhost Of Tabor Wipe... | Rumble LIVE
27.2K1