Premium Only Content
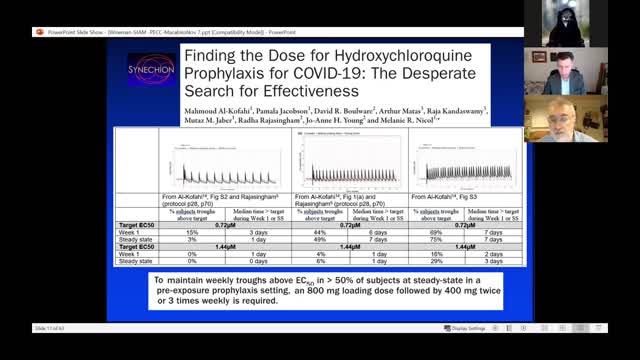
Dr. David Wiseman's presentation at the C19 minisymposium session, November 7 2021
Re-analysis of policy-shaping studies on hydroxychloroquine and ivermectin reverses original findings to yield significant benefits. Review in the context of regulatory decisions on vaccines.
Abstract: It was with skepticism about the efficacy of hydroxychloroquine (HCQ) in Covid-19, that we read a paper reported in NEJM that was one of only two substantive papers cited by FDA in its revocation of the EUA for HCQ in June 2020. This was a study of postexposure prophylaxis (PEP) in persons experiencing a high or moderate risk exposure to Covid-19. Subjects enrolled in the study online and received HCQ or a folate placebo with the goal of preventing development of disease. Data presented in the supplemental appendix suggested, surprisingly, that the drug may have some efficacy in certain subpopulations. After contacting the PI, he pointed out a possible benefit of very early intervention. Exploring this observation, an examination of the raw dataset suggested that the intervention lag had likely been underestimated. After further data requests, we established that $52\%$ of subjects had received drug later than the overnight delivery assumed by the investigators. Correcting for this error yielded a $42\%$ reduction $(p=0.044)$ in Covid-19 compatible disease associated with HCQ given $\leq 3$ days from exposure. We found a similar problem in a companion study performed by the same team under the same logistical arrangements addressing early symptomatic subjects. We were unable to obtain corrected intervention lag data to determine if effects could be observed that were greater than the $20\%$ reduction of symptomatic Covid-19 reported originally. We also re-analyzed a study of early treatment using ivermectin (IVM) reported in JAMA in March 2021. The paper reported several execution errors or changes, notably the dosing of some placebo subjects with active drug, and the use of different types of placebos in different trial phases. Obtaining the raw dataset, accounting for these issues yielded a $56\%$ reduction $(p=0.033)$ of residual Covid-19. We will discuss these studies in the context of significant decisions made during the pandemic, particularly in light of recent decisions made by FDA and CDC regarding the Covid-19 vaccines.
Presented at the "Efficacy and safety statistics of COVID-19 treatment and prophylaxis protocols" minisymposium session at the 4th Annual Meeting of the SIAM Texas-Louisiana Section on November 7, 2021. This presentation does not necessarily represent the opinions or policies of SIAM, but it is protected under Academic Freedom in Research and the 1st Amendment of the United States Constitution.
-
 2:50:01
2:50:01
Eleftherios Gkioulekas -- Research and Commentary
2 years agoExpert testimony to Pennsylvania State Senate, June 2023
224 -
 LIVE
LIVE
Midnight In The Mountains™
4 hours agoHappy Christmas Eve! Arc Raiders before the Family Dinner this Evening Hop in and Say Hello!
39 watching -
 1:58:38
1:58:38
Nikko Ortiz
5 hours agoMilitary Submarines And Soldiers Going AWOL... | Rumble LIVE
41.5K11 -
 1:08:07
1:08:07
Sean Unpaved
6 hours agoChristmas Eve Special With Sean Salisbury! | UNPAVED
31.4K4 -
 1:31:26
1:31:26
Film Threat
1 day agoCHRISTMAS EVE CHILL STREAM! SANTA'S NAUGHTY LIST OF MOVIES! | Hollywood on the Rocks
21.1K2 -
 56:57
56:57
MYLUNCHBREAK CHANNEL PAGE
6 hours agoA.I. EXPOSING the Fake History
24.6K17 -
 30:40
30:40
Tudor Dixon
6 hours agoCissie Graham Lynch on Faith, Family, & Christmas | The Tudor Dixon Podcast
17.3K4 -
 1:57:31
1:57:31
Badlands Media
15 hours agoBadlands Daily – 12/24/25: Christmas Eve Rants, Pharma Scams, and Saving Christmas
131K18 -
 3:17:34
3:17:34
LumpyPotatoX2
5 hours agoWar Thunder: Infantry CBT - Gaijin Partner
26.8K3 -
 2:59:56
2:59:56
Wendy Bell Radio
11 hours agoTDS Is A Bad Color On Everyone
133K151