Premium Only Content
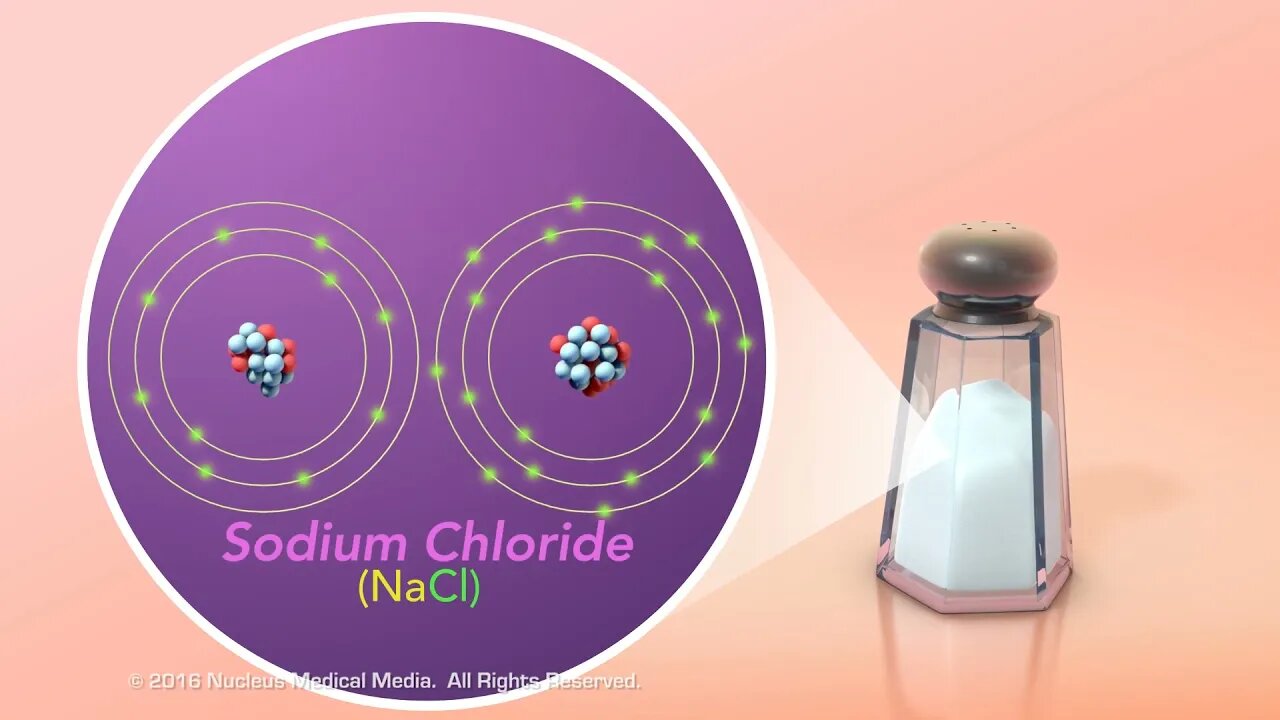
Ionic vs. Covalent Bonds
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#ChemicalBonds #IonicBonds #CovalentBonds
SCIENCE ANIMATION TRANSCRIPT: In this video, we will compare ionic and covalent bonds. In order to understand ionic bonds, we need to talk about ions first. Atoms are electrically neutral because they have equal numbers of both positively charged protons and negatively charged electrons. However, an atom can become a charged particle called an ion if it gains or loses electrons. If an atom gains electrons, it acquires more negative charge. As a result, it becomes a negatively charged ion. Conversely, if an atom loses electrons, it loses some of its negative charge and becomes a positively charged ion. The only way to get a positively charged ion is to lose negatively charged electrons. Remember, you can't just add a proton to make a positive ion because changing the number of protons would change it into a different element. Now, let's talk about ionic bonds. Notice that this term contains the word ion. That's because ionic bonds create ions out of electrically neutral atoms by the transfer of one or more valence electrons from one atom to another. Further, electrically neutral atoms of elements whose outer shell is less than half filled with valence electrons tend to donate electrons, while atoms whose outer shell is more than half filled tend to attract electrons. For example, sodium and chlorine atoms are electrically neutral. Chlorine, which only needs one electron to fill its outer shell, strongly attracts sodium's single valence electron. So, these elements react to form a chemical bond, creating sodium chloride. Sodium chloride, otherwise known as table salt, is an example of an ionically bonded compound. This is because the electrically neutral sodium atom became a positively charged ion by losing its valence electron. And chlorine became a negatively charged ion by gaining this electron from sodium. So, how do covalent bonds occur? The simplest substance that contains a covalent bond is a molecule of hydrogen gas also known as H2. A hydrogen atom has only one electron in its outer shell, which for this atom is also the shell nearest the nucleus. This shell can hold a maximum of two electrons. So, atoms of hydrogen tend to pair up and share their electrons so that both atoms have their outer shell filled. As you can see, covalent bonds occur when atoms share pairs of electrons. In this molecule, the hydrogen atoms form a single covalent bond. Another example of a covalently bonded molecule is carbon dioxide, or CO2. From its chemical formula, you know that carbon dioxide contains one carbon atom and two oxygen atoms. Carbon has four valence electrons, and both oxygen atoms have six valence electrons. But all three atoms would need eight electrons to fill their outer shells. So, each oxygen atom shares a pair of electrons with the pair of electrons in carbon. This results in two double covalent bonds where two pairs of electrons are shared between each atom. To summarize, the two main types of chemical bonds are ionic bonds and covalent bonds. In ionic bonds, one or more electrons are transferred from one atom to another. In covalent bonds, one or more pairs of electrons are shared between atoms. [music]
NSV16029
-
 LIVE
LIVE
LFA TV
13 hours agoLIVE & BREAKING NEWS! | FRIDAY 2/13/26
2,410 watching -
 1:02:55
1:02:55
Crypto Power Hour
13 hours ago $2.00 earnedBlockchain Fundamentals The Shared Tamper Proof Ledger
24.9K7 -
 1:03:38
1:03:38
Chris Ripa
13 hours agoTHE WAKE UP CALL - 02/13/2026 - Episode. 63
21.8K -
 21:17
21:17
Cash Jordan
18 hours ago"YOU KNEW EVERYTHING"... Epstein Hearings IMPLODE as 'Coverup' BACKFIRES on LIVE TV
24.8K32 -
 12:04
12:04
TacticalTurkeys
14 hours agoRuger Precision .308 X Surefire RC2 Beck Ammunition 168gr Subsonic Silent Operator.
22.9K9 -
 2:05:16
2:05:16
BEK TV
1 day agoTrent Loos in the Morning - 2/13/2026
31K -
 12:16
12:16
Anthony Rogers
16 hours agoEpisode 404 - Story of the Year
22.8K2 -
 1:18:56
1:18:56
Triggernometry
1 day agoIs This The End Of Humanity?- Eric Weinstein
40.9K20 -
 11:38
11:38
Clyde Do Something
12 hours ago $7.01 earnedCanada’s Police Is a MASSIVE JOKE Pronouns are The PRIORITY
64.5K35 -
 1:13:54
1:13:54
Dinesh D'Souza
2 days agoEveryone is Wrong About Anti-Semitism
27.3K97