Premium Only Content
Overview of Chemical Bonds
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#ChemicalBonds #IonicBonds #CovalentBonds
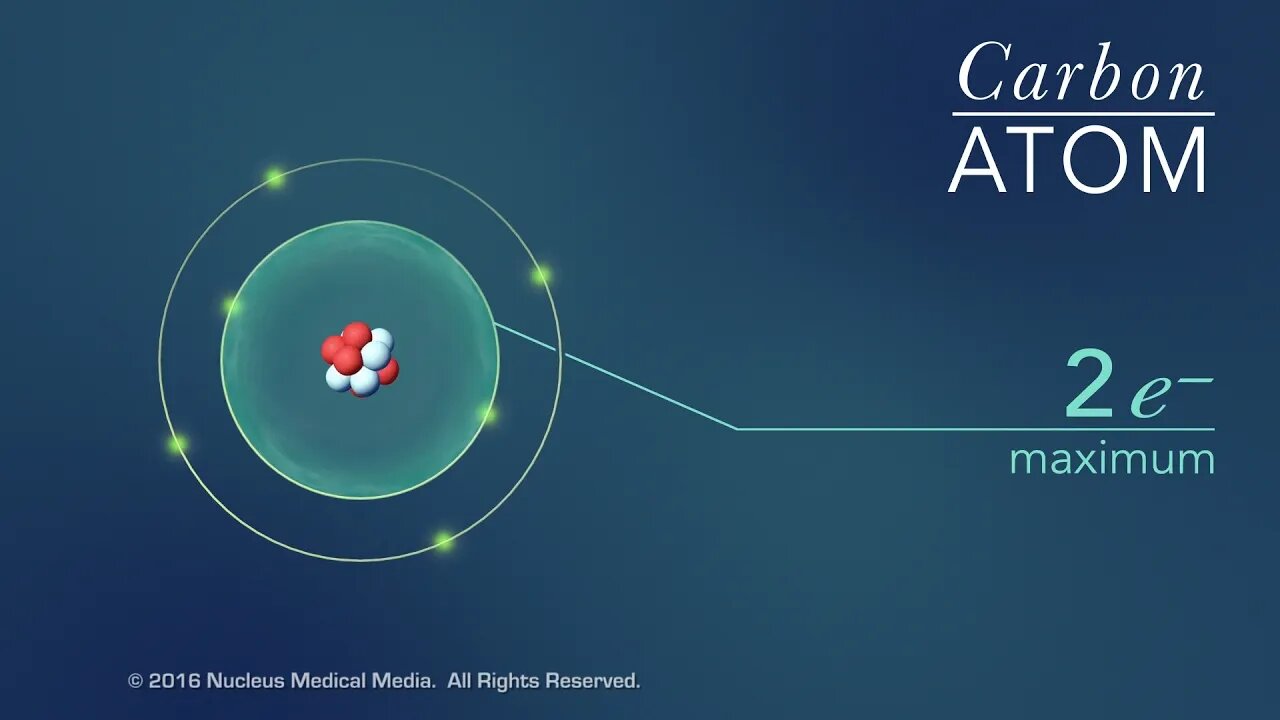
SCIENCE ANIMATION TRANSCRIPT: This video is an overview of chemical bonds. How do the atoms of elements form chemical bonds? Recall that electrons and an atom surround the nucleus. They feel energy levels or shells in specific numbers. Electrons in an atom's inner shells are commonly referred to as core electrons. Core electrons don't participate in chemical bonds. In contrast, electrons in the outermost shell of an atom are called valence electrons. Valence electrons do participate in forming chemical bonds. For example, a carbon atom with six protons and six neutrons in the nucleus has six electrons. Notice that carbon has both core and valence electrons. The innermost shell of any atom can hold a maximum of two electrons and the next shell can hold up to eight electrons. So, two of the electrons in carbon fill the first shell, and the remaining four electrons are in the next shell. The two electrons in the first shell are carbon's core electrons. The four electrons in the outer shell are carbon's valence electrons. Atoms with fewer valence electrons than its outer shell can hold aren't as stable as atoms with full outer shells. However, these atoms can become more stable if their outer shell is filled. This can happen either by loosing electrons to another atom or attracting electrons from another atom. This interaction of valence electrons between atoms results in the formation of chemical bonds. Elements that have completely filled outer shells such as helium are mostly non-reactive which means, they don't usually form chemical bonds. Why is that? Well, it's because their outer shells can't accept anymore electrons and losing all of their valence electrons requires too much energy. There are two main types of chemical bonds, they are ionic bonds, when electrons are transferred from one atom to another, and covalent bonds, when atoms share electrons. We'll discuss this in more detail separately. [music]
NSV16020
-
 1:44:01
1:44:01
Tucker Carlson
5 hours agoTucker Carlson on the Israel First Meltdown and the Future of the America First Movement
49.2K387 -
 4:02:08
4:02:08
Alex Zedra
5 hours agoLIVE! Phasmaphobia New Map!
41.2K2 -
 2:16:06
2:16:06
Laura Loomer
7 hours agoEP155: Jihad Makes Its Move On The White House
37.7K53 -
 2:18:47
2:18:47
TheSaltyCracker
7 hours agoDem's Epstein Drop Backfires ReeEEStream 11-12-25
103K211 -
 17:08
17:08
Demons Row
7 hours ago $4.81 earnedMost Dangerous Motorcycle Clubs That Ever Existed 💀🔥
35.1K3 -
 12:51
12:51
The Gun Collective
8 hours agoWOW! -- LOTS of new GUNS just came out!
17.3K10 -
 2:06:53
2:06:53
I_Came_With_Fire_Podcast
15 hours agoWhat IS America First | Al Qaeda in the White House | China's Spy Highway
14.4K4 -
 1:46:55
1:46:55
Adam Does Movies
10 hours ago $0.71 earnedTalking Movies + Ask Me Anything - LIVE
9.46K -
 1:30:33
1:30:33
Glenn Greenwald
9 hours agoMAGA Outrage Over Trump's Plan for More H-1B Visas: With Prof. Ron Hira; Latest Epstein/Israel Revelations and Newly Released Emails: With Drop Site's Murtaza Hussain | SYSTEM UPDATE #546
126K44 -
 3:39:09
3:39:09
Barry Cunningham
9 hours agoBREAKING: PRESIDENT TRUMP DINNER | GOVERNMENT SHUTDOWN VOTE | MAHA SUMMIT WITH RFKJR & JD VANCE!
49.2K38