Premium Only Content
Elements
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#elements #AtomicNumber #Chemistry
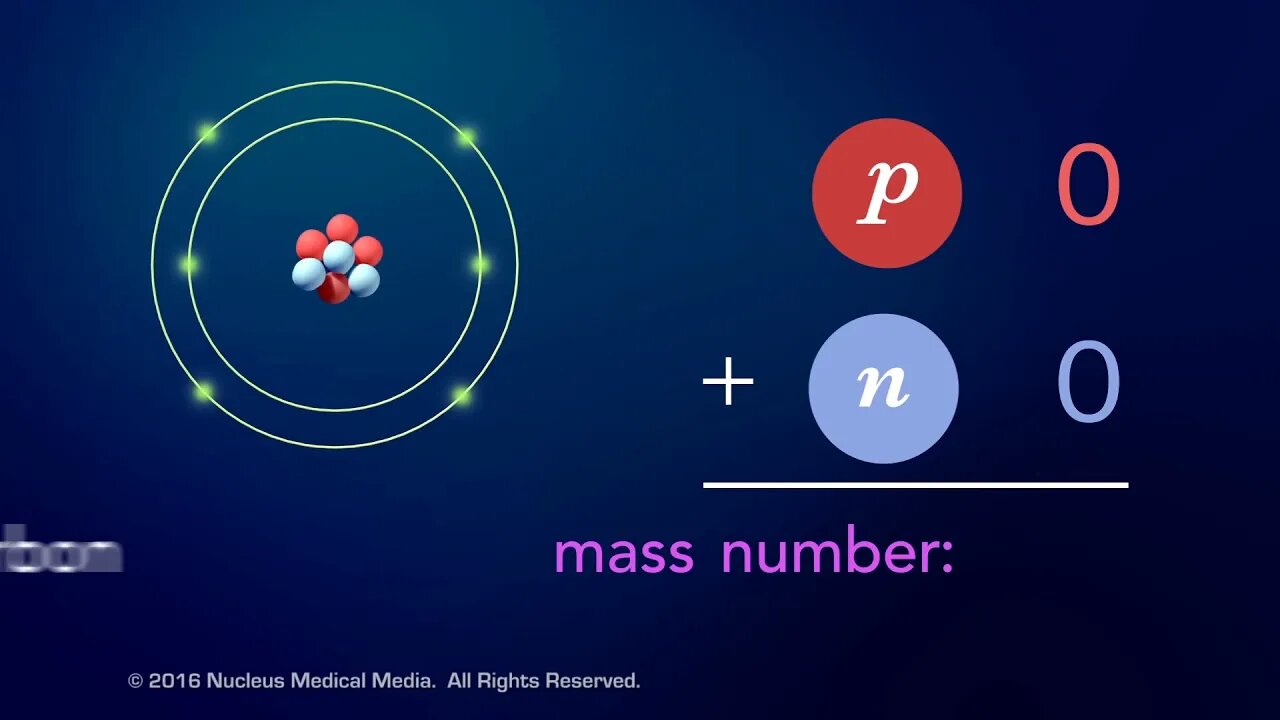
SCIENCE ANIMATION TRANSCRIPT: What is an element? Elements are pure substances that are made up of only one type of atom such as hydrogen, carbon, or mercury. So, what makes one element different from another? Well, it's the number of protons in a single atom of an element. This is called the element's atomic number. For example, hydrogen has one proton in its nucleus, so its atomic number is one. Carbon has six protons, so its atomic number is six. And mercury's atomic number is 80 because it has 80 protons in its nucleus. Since the nucleus contains almost the entire mass of an atom, the number of particles it contains has a big effect on the atom's mass. Each positively charged proton has a mass unit of one, and each neutral neutron also has a mass unit of one. The total number of protons and neutrons in the nucleus of an atom is called the mass number. In this example, the mass number of a hydrogen atom is one. Hydrogen is the only element that usually doesn't have any neutrons. The mass number of this carbon atom with six protons and six neutrons is 12. And the mass number of this mercury atom with 80 protons and 121 neutrons is 201. Needless to say, mercury is a match heavier element than hydrogen or carbon. Even though every atom of the same element always has the same number of protons, sometimes an element has atoms with different numbers of neutrons. Ordinary hydrogen has no neutrons, but there's a version of hydrogen with one neutron and another version with two neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. The three isotopes you see here are all still hydrogen because they all have only one proton. Since neutrons have about the same mass as protons, isotopes of the same element have different mass numbers. In fact, an element's isotopes are often identified by their mass numbers. To sum up, an element is a pure substance made of atoms that always have the same number of protons. This means atoms with different numbers of protons are different elements. The number of protons in one atom of an element is called the atomic number. The number of protons plus neutrons in one atom is called the mass number. Isotopes are atoms of the same element with different numbers of neutrons. [music]
NSV16021
-
 1:32:32
1:32:32
Graham Allen
2 hours agoWill Epstein Cost Us the MIDTERMS??! + It's a New World and It's Time For Us To Fight the RIGHT Way!
141K228 -
 20:03
20:03
Clintonjaws
11 hours ago $1.52 earnedTV Host Explodes At Woke Guest For Defending Men on Women's Toilets!
4.01K14 -
 LIVE
LIVE
Caleb Hammer
3 hours agoThe Most Delusional Woman In Financial Audit History
221 watching -
 LIVE
LIVE
The Big Mig™
2 hours agoTo The Moon Or Bust, Elon Musk Says We're Going To The Moon
3,105 watching -
 1:04:26
1:04:26
BonginoReport
13 hours agoDems Hold Senate Hostage Over ICE Funding | Episode 225 – (02/13/26) VINCE
137K77 -
 1:10:37
1:10:37
Chad Prather
16 hours agoClear Eyes, Clean Hearts: The Kingdom Way of Judgment
83.9K34 -
 LIVE
LIVE
Badlands Media
8 hours agoBadlands Daily: 2/13/26
3,485 watching -
 1:26:40
1:26:40
Connor Tomlinson
6 hours ago“Starmer Has Blood on His Hands”
6.86K3 -
 1:58:31
1:58:31
The Chris Salcedo Show
19 hours ago $6.46 earnedWhile Posers Fold, Conservative Patriots Keep Fighting & Winning For Liberty
30.8K8 -
 1:02:55
1:02:55
Crypto Power Hour
14 hours ago $2.67 earnedBlockchain Fundamentals The Shared Tamper Proof Ledger
35.5K11