Premium Only Content
New experimental mRNA trial suspended
Vaccines and Related Biological Products Advisory Committee December 12, 2024 Meeting Announcement
Entire briefing document
https://www.fda.gov/media/184301/download
Pediatric RSV vaccine trial enrollment on hold in US, VRBPAC says
The halt follows a severe respiratory disease safety signal observed in a July 2024 phase 1 trial of Moderna's mRNA-1345 and mRNA-1365 vaccine candidates.
A phase I trial
The effects of the medication on about 20 to 80 healthy volunteers
To evaluating safety and ideal dosage
70% move on to phase II
This study, safety, tolerability, and immunogenicity
Who were the subjects
Children aged younger than 2 years
Respiratory syncytial virus (RSV) -naïve children aged 2 through 5 years
The halt of this mRNA RSV vaccine
Severe respiratory disease safety signal
Safety signal led to study pause
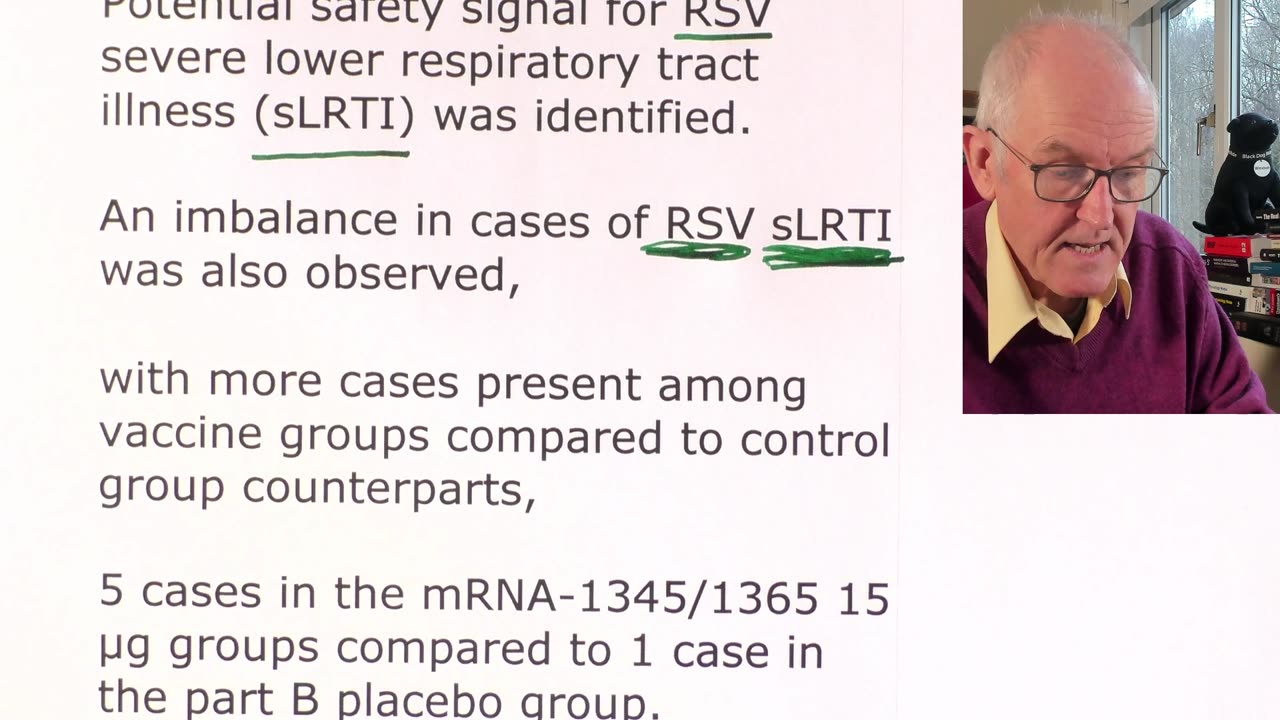
Potential safety signal for RSV severe lower respiratory tract illness (sLRTI) was identified.
An imbalance in cases of RSV sLRTI was also observed,
with more cases present among vaccine groups compared to control group counterparts,
5 cases in the mRNA-1345/1365 15 µg groups compared to 1 case in the part B placebo group.
Of these 6 cases, 5 required hospitalization, including 1 infant who required mechanical ventilation.
which raised concern for vaccine-associated enhanced respiratory disease.
The protocol’s study pause criterion of any sLRTI with positive polymerase chain reaction (PCR) for RSV in ≥2 participants was met.
One, two and three dose parts planned
Part A (Cohorts 1 and 2)
30 µg mRNA-1345, 30 µg mRNA-1365, and placebo
approximately 90 participants 8 months to <24 months of age
(randomized in a 1:1:1 ratio, respectively)."
"Part B (Cohorts 3 through 6)
2 dose levels of mRNA-1345 and mRNA-1365 and placebo
Approximately 120 participants 5 months to <8 months of age
(randomized in a 1:1:1 ratio, respectively)
Part C (Cohorts 7 and 8)
3 doses of 30 µg mRNA-1345
Approximately 100 participants 8 months to <12 months of age
who have (Cohort 7) or have not (Cohort 8) previously received nirsevimab.
VRBPAC meeting and outlook
Vaccines and Related Biological Products Advisory Committee
Vaccine-associated enhanced respiratory disease
Currently considering criteria for recommencement
Scam Accounts
https://www.facebook.com/Campbellteaching/
https://www.facebook.com/dr.john.campbell/
Genuine Campbell Teaching Accounts
https://substack.com/@johninengland?utm_source=user-menu
https://www.paypal.com/mep/dashboard
https://www.paypal.com/donate/?cmd=_s-xclick&hosted_button_id=78GGHGLK5ZXAE
-
 22:42
22:42
Dr. John Campbell
14 days agoPCR was usually wrong
12K96 -
 3:24:48
3:24:48
VapinGamers
6 hours ago $25.39 earnedDestiny 2 - Dungeons and Loot with Friends - !rumbot !music
84.1K1 -
 2:07:44
2:07:44
TimcastIRL
7 hours agoTrump's Secret Plan To Make Charlie Kirk VP, America Fest IN CIVIL WAR | Timcast IRL
213K178 -
 4:09:13
4:09:13
I_Came_With_Fire_Podcast
16 hours agoLive Fire: Christmas Special
34.1K7 -
 46:26
46:26
Sarah Westall
9 hours agoWhat’s Behind the Silver Surge? Large Institutions Cashing In w/ Andy Schectman
34K2 -
 6:42:10
6:42:10
Turning Point USA
15 hours agoLIVE NOW: AMFEST DAY 2 - VIVEK, JACK POSOBIEC, MEGYN KELLY, ALEX CLARK AND MORE…
1.28M180 -
 1:14:37
1:14:37
Flyover Conservatives
1 day agoHow to Win 2026 Before It Starts — Clay Clark’s Goal-Setting Blueprint | FOC Show
35.5K1 -
 12:52
12:52
The Kevin Trudeau Show Limitless
2 days agoBeyond Good And Bad: The Hidden Reality Code
77.8K21 -
 1:03:11
1:03:11
BonginoReport
11 hours agoBrown U Security Failures EXPOSED - Nightly Scroll w/ Hayley Caronia (Ep.201)
155K47 -
 51:09
51:09
Patriots With Grit
6 hours agoWill A.I. Replace Doctors? | Dr. Stella Immanuel MD
24.2K4